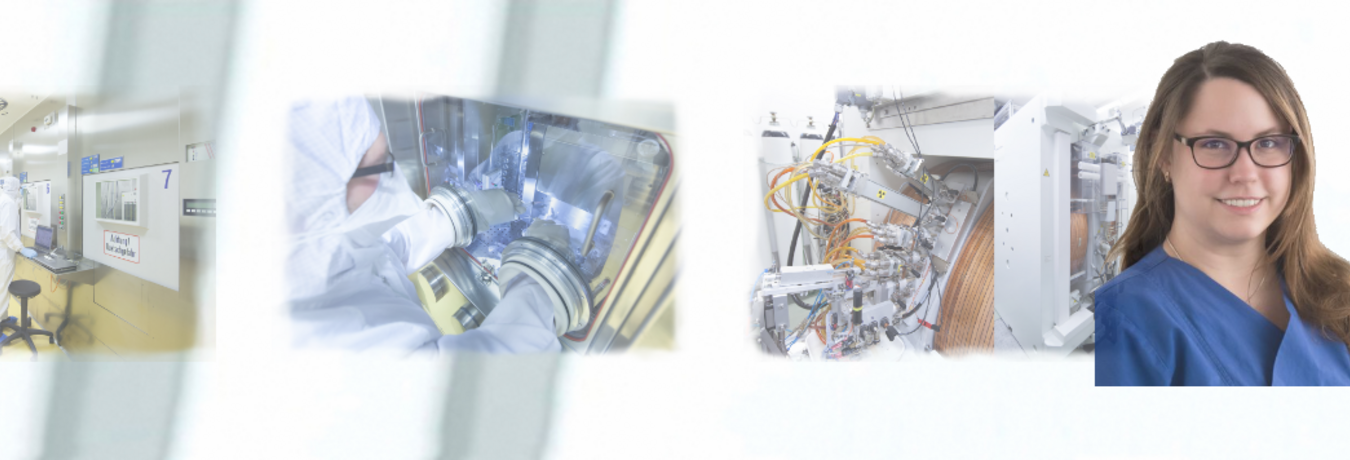
The Radiopharmacy/Cyclotron Unit of the Department of Nuclear Medicine at Ulm University Hospital is a highly specialized unit in which radiopharmaceuticals labeled with short-lived positron emitters are produced for nuclear medical diagnostics using PET or PET/CT. In addition, state-of-the-art radiotherapeutics are also produced for targeted radionuclide therapy.
Production of short-lived positron emitters
The half-lives of the radionuclides used are so short (between 2-110 min) that it is absolutely necessary to produce these radionuclides oneself using one's own particle accelerator. For radionuclide production, we operate a PETtrace cyclotron (16.5 MeV protons, 8.25 MeV deuterons; General Electric) and a Ge-68/Ga-68 generator.



The radionuclide production comprises the radiochemical conversion of the generated radionuclides with the corresponding pharmaceutical precursor compounds to form the radiopharmaceutical. The Radiopharmacy/Cyclotron department has a state-of-the-art clean room for GMP-compliant radiopharmaceutical production.
There, seven fully shielded lead cells ("hot cells") are available in the radiopharmaceutical production area. Thus PET radiopharmaceuticals for human use (patient care and clinical research) are produced in parenteral form under clean room conditions. Sterile filling and portioning of the "radiotracers" takes place in a radiopharmaceutical isolator. The principles and official requirements regarding pharmaceutical production and radiation protection must always be observed and complied with. In particular, the focus is on testing the pharmaceutical quality. This is verified in a comprehensive quality control (identity, chemical and radiochemical purity, sterility, tonicity, pH value, bacterial endotoxins).
The work and activities to be performed in this area are logistically extremely complex and must be carried out particularly effectively. Therefore, this demanding work is particularly challenging. The deployment of an experienced and highly specialized team is essential for this.
Diagnostic radiopharmaceuticals (PET radiopharmaceuticals)
The following PET radiopharmaceuticals are regularly produced for clinical use:
- [18F] FDG (Tumors, inflammation, dementia)
- [68Ga] PSMA-Ligand (Prostate Cancer)
- [68Ga] DOTA-i-TATE (neuroendocrine tumors, meningiomas)
- [11C] PIB (Detection of amyloid deposits in dementias.)
- [11C] Methionin (Amino acid metabolism, parathyroid gland, brain tumors)
The approved medicinal product [18F] FDG (“Glucopet-18F“) is produced daily for diagnostics (glucose metabolism: especially tumors, inflammation, dementia) using PET/CT.
In addition, it is also possible to offer other PET radiopharmaceuticals required for special problems at short notice (a.o. [11C] Cholin, [18F] Fluorid, [68Ga] Pentixafor).
Therapeutic radiopharmaceuticals
The Radiopharmaceuticals division also produces therapeutic radiopharmaceuticals for modern nuclear medicine therapy (endoradiotherapy).
In particular, the focus is on the effective treatment of neuroendocrine tumors and metastatic prostate carcinoma. The following radiotherapeutics are produced for this purpose:
- [177Lu] DOTA-i-TATE
- [177Lu] PSMA I&T
Research
The division also has a radionuclide research laboratory in which radiopharmaceutical/radiochemical development/research is carried out in four radionuclide fume hoods.
One focus here is radiolabelling using 89Zr for nanoparticles and antibodies for modern tumor diagnostics with PET.
For further information please click here.
Contact us
Address:
University Hospital Ulm
Clinic for Nuclear Medicine
Albert-Einstein-Allee 23
89081 Ulm
Germany
You can contact us by phone:
Mon - Thu: 08:00-12:00 and 13:00-16:00
Fr: 08:00-14:00
Contact person:
Dr. Christoph Solbach | Head of Radiopharmacy / Cyclotron