Chronic stress is a burden of modern societies and is known to be a risk factor for numerous somatic and affective disorders, including cardiovascular dysfunction, inflammatory bowel disease, gastric ulceration, general and/ or social anxiety- and depression-related diseases, as well as systemic immunological dysfunctions. Although many of these disorders are paralleled by either hypocorticism, glucocorticoid (GC) resistance, or a combination of both, resulting in a decreased GC signalling, the underlying aetiology of these disorders is still poorly understood, at least partly due to a lack of appropriate and clinically relevant animal models.
Importantly, not all people are equally affected by chronic stressors. While some individuals subjected to severe adverse life events develop pathologies, others do not. But again, the underlying mechanisms engendering resilience and vulnerability are also far from being understood and, thus, the possibility to clinically benefit from these observations is currently still limited.
A failure of immunoregulation, attributable to reduced exposure to the microbial environment within which the mammalian immune system evolved ("Old friends hypothesis"), is thought to be one factor contributing to recent increases in stress-related chronic inflammatory disorders, as well as mood disorders for which chronic low-grade inflammation is a risk factor ("cytokine theory of depression") (Lowry et al., 2016 Curr Environ Health Rep; Reber et al., 2016 PNEC).
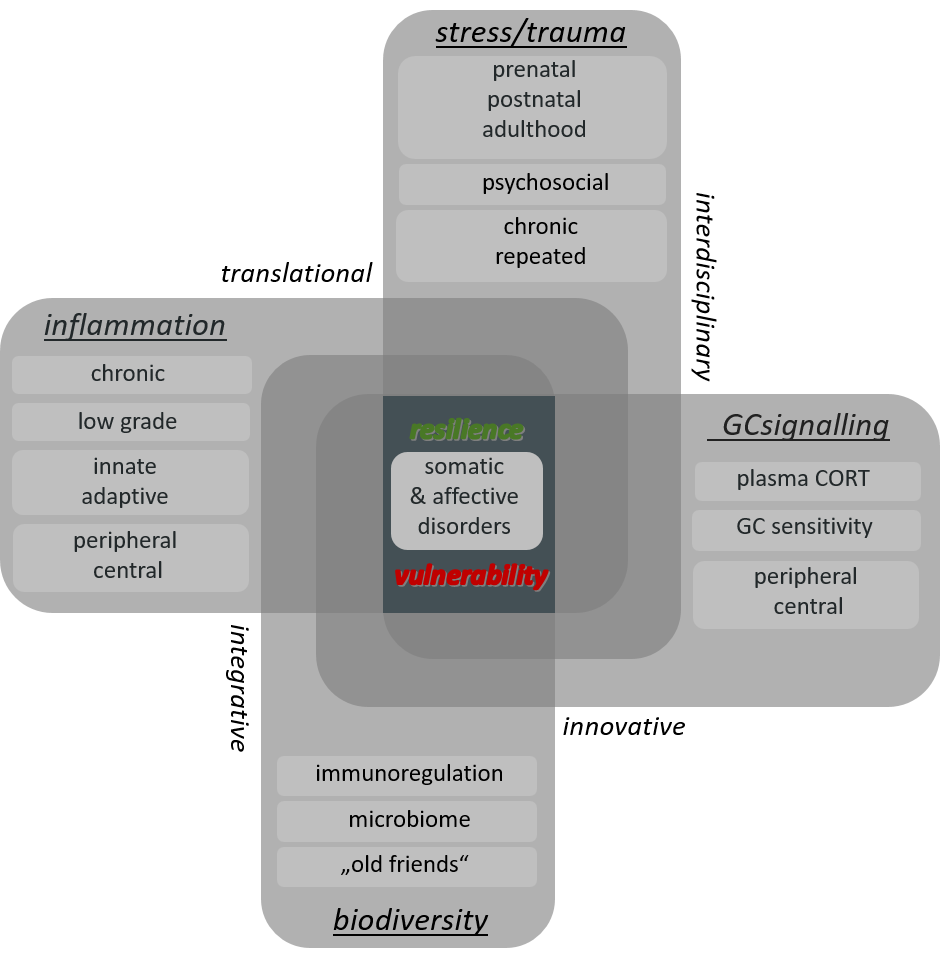
Therefore, the main research aims of the Laboratory for Molecular Psychosomatics are to extend the current knowledge on the mechanisms underlying 1i) stressor-induced development of somatic and mental pathologies and 1ii) individual differences in stress resilience and 2) to use this mechanistic knowledge for the development of novel strategies in terms of stress protection (Fig. 1).
Funding Identity of the Laboratory for Molecular Psychosomatics

Effects of Lactobacillus reuteri DSM 17938 on the acute psychological, endocrine and immunological stress response in healthy city dwellers. (German Research Foundation, RE 2911/26-1; successfully applied for by S.O. Reber).
This partnership aims to build research capacities, foster long-term collaboration, and lay the groundwork for raising future international funding for exploring how brain-body integration, circadian rhythms, and environmental stress influence immune function. (successfully applied for by Prof. Dr. Ilia Karatsoreos (Department of Psychological and Brain Sciences at University of Massachusetts Amherst, USA) and Prof. Dr. Stefan Reber at Ulm University Medical Center).

The role of circulating small extracellular vesicles in chronic psychosocial stress/ PTSD- related inflammation. (German Research Foundation, LA 5673/3-1; successfully applied for by D. Langgartner).

Investigating the role of circulating small extracellular vesicles in chronic psychosocial stress-/ PTSD-related inflammation. (Collaborative Research Consortium (CRC) 1149/ German Research Foundation; successfully applied for by Dr. D. Langgartner)

Effects of early life stress on bone homeostasis and fracture healting. (German Research Foundation, Project B06 within the CRC 1149, INST 40/599-1; successfully applied for by S.O. Reber and M. Haffner-Luntzer (Co-PI, Institute of Ofthopaedic Research and Biomechanics, Ulm University)).

Mycobacterium vaccae immunization: Inducing resilience to stress during pregnany in the dam and protecting her offspring. (German Research Foundation, RE 2911/23-1; successfully applied for by S.O. Reber Ulm and D.A. Slattery (Co-PI, SL141/6-1, Laboratory for Translational Psychiatry, Department of Psychiatry, Psychosomatics and Psychotherapy, Goethe University Frankfurt))

Facilitating stress resilience by "Old friends": Immunization with Mycobacterium vaccae prevents the negative effects of early life stress on chronic stress vulnerability during adulthood. (German Research Foundation, RE 2911/21-1; successfully applied for by S.O. Reber)
Stress-protective effects of Mycolicibacterium aurum Aogashima. (successfully applied for by S.O. Reber)

Facilitating resilience to repeated hits of psychosocial traumatization by "Old friend" organisms – a closer look at the influence of the intestinal microbiome. (Collaborative Research Consortium (CRC) 1149/ German Research Foundation; successfully applied for by D. Langgartner)

Effects of psychosocial trauma on bone homeostasis and fracture healing. (German Research Foundation, Project B06 within the CRC 1149, INST 40/599-1; successfully applied for by S.O. Reber Ulm and M. Haffner-Luntzer (Co-PI, Institute of Orthopaedic Research and Biomechanics, Ulm University))

Ph.D. stipend for Sandra Förtsch from April to December 2018. (International Graduate School in Molecular Medicine of Ulm University; successfully applied for by S.O. Reber)

Promotion of stress resilience by non-invasive immunoregulatory approaches.' (Office of Naval Research Global, N00014-17-S-B001; Grant12274897; successfully applied for by S.O.Reber)

Additive effects of multiple psychosocial traumatic life experiences on subsequent blunt thorax trauma. ("Bausteinprogramm" of the medical faculty of the Ulm University; successfully applied for by D. Langgartner)

Danger response and regeneration following musculoskeletal trauma: interdisciplinary approach to link physical and psychological injuries. (Innovation fond of the medical faculty of the Ulm University; successfully applied for by S.O. Reber)

Mechanisms underlying CSC-induced breakdown of HPA axis functions. (German Research Foundation, RE 2911/5-1; successfully applied for by S.O. Reber)

Effects of psychosocial stress on intestinal homeostasis and mechanisms underlying stress-induced colitis. (German Research Foundation, RE 2911/2-1; successfully applied for by S.O. Reber)
Research Profile of the Laboratory for Molecular Psychosomatics
To investigate our research hypotheses stated above, we employ several preclinically validated rodent models of chronic psychosocial stress and study in detail their effects on behavioral, physiological, neuroendocrine, and immunological parameters, thereby considering individual differences in the stress coping strategy. Our main paradigm in this context to induce chronic psychosocial stress during adulthood is the chronic subordinate colony housing (CSC) paradigm (Reber et al., 2007 PNEC; Reber, 2012 PNEC; Langgartner et al., 2015 Frontiers in Psychiatry; Reber et al., 2016 PNEC). The CSC model is based on repeated psychosocial traumatization (social defeat, Days 1, 8, and 15) and prolonged (19 d) social subordination of male experimental mice to a larger dominant male and results in many behavioral, endocrine and immunological changes known from posttraumatic stress disorders patients (for review see Reber et al., 2016 PNEC). The value and clinical relevance of this stress model has recently been acknowledged with the "Curt P. Richter Award" by the International Society for Psychoneuroendocrinology (ISPNE). To induce chronic psychosocial stress in mice during early life we employ the maternal separation (MS) paradigm (Veenema et al., 2008 Endocrinology). Here, the pubs are separated from the mother for 3h per day between postnatal days (PND) 1-14. MS negatively affects the behavior (i.e. increases anxiety-related behavior), alters HPA axis (re)activity, and promotes immune activation, which is in line with what has been reported in humans exposed to early life trauma/stress.
To induce acute psychosocial stress in human volunteers under standard laboratory conditions for investigating its effects on mood, physiology and the immune system, we employ a slightly modified version of the "Trier Social Stress Test (TSST)", originally described earlier (Kirschbaum et al., 1993 Neuropsychobiology). TSST exposure reliably activates the two main stress axes, namely the sympathetic nervous system (SNS) and the hypothalamus-pituitary-adrenal (HPA) axis, as well as the cardiovascular and the immune system.
Current Research Projects
P1
Immunoregulatory approaches to promote stress resilience
It is the major aim of this preclinical project, to promote resilience against stress-related somatic and affective pathologies in male and female mice by repeated subcutaneous (s.c.), intranasal (i.n.) or intragastric (i.g.) administrations of heat-inactivated preparations of immunoregulatory "old friends from mud and soil", including Mycobacterium vaccae NCTC 11659, M. vaccae ATCC 15483typestrain and M. aurum DSM 33539, and to understand the underlying mechanisms.
Background: Epidemiological data provide strong evidence for a steady rise in the incidence of many stress-associated psychosomatic disorders in developed countries since the 1950s.(1-10) Although the underlying mechanism is not clear, decreased immunoregulation, resulting from decreased numbers of regulatory T cells (Tregs),(11, 12) is likely to play a role. Furthermore, as less people in rural compared with urban areas suffer from stress-associated somatic and mental disorders,(13-19) it is likely that a reduced exposure, especially during early life,(20) to microorganisms with which mammals co-evolved (=Old Friends), at least in part accounts for immunoregulatory deficits, increased stress-induced inflammation and disease prevalence in modern industrialized urban areas.
Main findings: Consistent with what is proposed by the “Old Friends” hypothesis, we and others have shown that repeated s.c. administrations with a heat-killed preparation of M. vaccae NCTC 11659, an abundant saprophytic “Old Friend” from mud with immunoregulatory properties, is effective in: i) stabilizing the gut microbiome,(21, 22) ii) increasing the percentage of Tregs in mesenteric lymph node cells,(21) iii) preventing stress-induced colitis and proinflammatory cytokine secretion from freshly isolated mesenteric lymph node cells stimulated with anti-CD3 antibody ex vivo,(21) iv) preventing stress-induced aggravation of dextran sulfate sodium (DSS)-induced colitis,(21) v) preventing stress-induced exaggeration of anxiety,(21) vi) preventing stress-induced microglial priming and neuroinflammation,(23-26) vii) ameliorating features of age‑associated microglia activation in the amygdala and hippocampus,(27) viii) preventing negative outcomes of sleep deprivation,(28) and ix) enhancing fear extinction.(29). In extension of these findings and in support of using “Old Friends” not only to prevent but also to treat stress-associated disorders, we recently showed that M. vaccae NCTC 11659 also ameliorates stress-induced anxiety when administered repeatedly via the s.c. route during chronic psychosocial stressor exposure, i.e., after the first psychosocial traumatization has occurred.(30) Own studies further confirm the stress protective effects of M. vaccae NCTC 11659 even when administered via the non-invasive i.n.(30) or i.g.(31) route, respectively. In detail, male mice administered i.g. with M. vaccae NCTC 11659 are protected against: 1) the stress-induced increase in splenic TLR2+ and TLR4+ polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) and TLR4+ monocytes/mononuclear (MO)-MDSCs; 2) the increase in functional splenic in vitro glucocorticoid (GC) resistance typically seen following psychosocial stress in combination with significant wounding (=physical trauma); as well as 3) the stress-induced increase in basal and LPS-induced splenic in vitro cell viability.(31)
In a recent study we further showed that the negative behavioral, immunological and physiological consequences of early life stress (ELA) induced by the maternal separation (MS) paradigm in both sexes, although relatively mild, are to a great extent prevented by subsequent s.c. M. vaccae NCTC 11659 administrations.(32) In extension, own data further show that the catabolic bone turnover and osteoporotic bone phenotype induced by ELA in female sex only was prevented by repeated s.c. administrations of M. vaccae NCTC 11659 (Schimmele et al., under review).
Importantly, the stress-protective effects of M. vaccae NCTC 11659 are not specific for this strain but transferable also to M. vaccae ATCC 15483T.(33) In support for the latter, also repeated i.g. administrations of female mice with M. vaccae ATCC 15483T prior to mating protected the male offspring of these females (i.e. intergenerational effects) from developing enhanced LPS-induced ex vivo cell viability of isolated splenocytes, increased delta cell viability (LPS-stimulated minus basal conditions) of isolated and ex vivo stimulated splenocytes in the absence of CORT as well as functional splenic ex vivo GC resistance as a consequence of CSC exposure during adulthood (Schiele et al., in preparation). As also repeated i.g. administrations of a heat-inactivated preparation of M. aurum DSM 33539 have profound stabilizing effects on the composition of the intestinal microbiome and were protective against the aggravating effects of stress on subsequent DSS colitis (Langgartner et al., under review), the stress protective effects reported for M. vaccae NCTC 11659 and M. vaccae ATCC 15483T seem to generalizable also to other nontuberculous mycobacteria (NTM) species.
Main collaborators: Prof. Dr. Christopher Lowry (University of Colorado, Boulder, USA), Prof. Dr. David Slattery (Goethe University Frankfurt), Prof. Dr. Steffen Stenger (Ulm University Medical Center, Ulm, Germany).
References:
1. Bach JF (2002): The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 347:911-920.
2. Woolcock A, Peat J (1997): Evidence for the increase in asthma worldwide. Ciba Found Symp. 206:122-134.
3. Williams H (1992): Is the prevalence of atopic dermatitis increasing? Clin Exp Dermatol. 17:385-391.
4. Upton MN, McConnachie A, McSharry C, Hart CL, Smith GD, Gillis CR, et al. (2000): Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan family study surveys of parents and offspring. BMJ. 321:88-92.
5. Rosati G, Aiello I, Mannu L, Pirastru M, Agnetti V, Sau G, et al. (1988): Incidence of multiple sclerosis in the town of Sassari, Sardinia, 1965 to 1985: evidence for increasing occurrence of the disease. Neurology. 38:384-388.
6. Poser S, Stickel B, Krtsch U, Burckhardt D, Nordman B (1989): Increasing Incidence of Multiple Sclerosis in South Lower Saxony, Germany. Neuroepidemiology. 8:207-213.
7. Group EAS (2000): Variation and trends in incidence of childhood diabetes in Europe. The Lancet. 355:873-876.
8. Farrokhyar F, Swarbrick E, Irvine E (2001): A critical review of epidemiological studies in inflammatory bowel disease. Scand J Gastroenterol. 36:2-15.
9. Smith K (2014): Mental health: A world of depression. Nature. 515:180-181.
10. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. (2018): Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 390:2769-2778.
11. Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, et al. (2010): Altered expression of CD4(+)CD25(+) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J Affect Disord. 124:68-75.
12. Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, et al. (2009): Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behavior and Immunity. 23:1117-1124.
13. Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. (2001): Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. The Lancet. 358:1129-1133.
14. Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. (2002): Environmental Exposure to Endotoxin and Its Relation to Asthma in School-Age Children. N Engl J Med. 347:869-877.
15. Ege MJ, Mayer M, Normand A-C, Genuneit J, Cookson WOCM, Braun-Fahrländer C, et al. (2011): Exposure to Environmental Microorganisms and Childhood Asthma. N Engl J Med. 364:701-709.
16. Peen J, Schoevers RA, Beekman AT, Dekker J (2010): The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand. 121:84-93.
17. Rook GA (2013): Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci U S A. 110:18360-18367.
18. Vassos E, Agerbo E, Mors O, Pedersen CB (2016): Urban–rural differences in incidence rates of psychiatric disorders in Denmark. The British Journal of Psychiatry. 208:435-440.
19. Pedersen C, Mortensen P (2001): Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 58:1039-1046.
20. Lynch SV, Boushey HA (2016): The microbiome and development of allergic disease. Curr Opin Allergy Clin Immunol. 16:165-171.
21. Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, et al. (2016): Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci U S A. 113:E3130-3139.
22. Foxx CL, Heinze JD, Gonzalez A, Vargas F, Baratta MV, Elsayed AI, et al. (2020): Effects of Immunization With the Soil-Derived Bacterium Mycobacterium vaccae on Stress Coping Behaviors and Cognitive Performance in a "Two Hit" Stressor Model. Frontiers in physiology. 11:524833.
23. Frank MG, Fonken LK, Watkins LR, Maier SF, Lowry CA (2018): Could Probiotics Be Used to Mitigate Neuroinflammation? ACS Chemical Neuroscience.
24. Frank MG, Fonken LK, Dolzani SD, Annis JL, Siebler PH, Schmidt D, et al. (2018): Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: Attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain, Behavior, and Immunity. 73:352-363.
25. Fonken LK, Frank MG, Gaudet AD, Maier SF (2018): Stress and aging act through common mechanisms to elicit neuroinflammatory priming. Brain, Behavior, and Immunity. 73:133-148.
26. Fonken LK, Frank MG, D'Angelo HM, Heinze JD, Watkins LR, Lowry CA, et al. (2018): Mycobacterium vaccae immunization protects aged rats from surgery-elicited neuroinflammation and cognitive dysfunction. Neurobiol Aging. 71:105-114.
27. Sanchez K, Darling JS, Kakkar R, Wu SL, Zentay A, Lowry CA, et al. (2022): Mycobacterium vaccae immunization in rats ameliorates features of age-associated microglia activation in the amygdala and hippocampus. Sci Rep. 12:2165.
28. Bowers SJ, Lambert S, He S, Lowry CA, Fleshner M, Wright KP, Jr., et al. (2020): Immunization with a heat-killed bacterium, Mycobacterium vaccae NCTC 11659, prevents the development of cortical hyperarousal and a PTSD-like sleep phenotype after sleep disruption and acute stress in mice. Sleep.
29. Hassell JE, Jr., Fox JH, Arnold MR, Siebler PH, Lieb MW, Schmidt D, et al. (2019): Treatment with a heat-killed preparation of Mycobacterium vaccae after fear conditioning enhances fear extinction in the fear-potentiated startle paradigm. Brain Behav Immun.
30. Amoroso M, Böttcher A, Lowry CA, Langgartner D, Reber SO (2020): Subcutaneous Mycobacterium vaccae promotes resilience in a mouse model of chronic psychosocial stress when administered prior to or during psychosocial stress. Brain Behav Immun. 87:309-317.
31. Langgartner D, Amoroso M, Kempter E, Kustermann M, Scheurer J, Lowry CA, et al. (2023): Mycobacterium vaccae protects against glucocorticoid resistance resulting from combined physical and psychosocial trauma in mice. Brain, Behavior, and Immunity. 109:221-234.
32. Mazzari G, Lowry CA, Langgartner D, Reber SO (2023): Subcutaneous Mycobacterium vaccae ameliorates the effects of early life adversity alone or in combination with chronic stress during adulthood in male and female mice. Neurobiology of Stress.100568.
33. Loupy KM, Cler KE, Marquart BM, Yifru TW, D'Angelo HM, Arnold MR, et al. (2021): Comparing the effects of two different strains of Mycobacterium vaccae, M. vaccae NCTC 11659 and M. vaccae ATCC 15483, on stress-resilient behaviors and lipid-immune signaling in rats. Brain, Behavior, and Immunity.
P2
Stress-induced glucocorticoid resistance
It is the major aim of this preclinical project to investigate and understand the mechanisms underlying the development of splenic ex vivo glucocorticoid (GC) resistance in male mice as consequence of psychosocial stress associated with physical trauma, as for instance severe bite wounds or planned surgery.
Background: Chronic psychosocial stress is a major burden of modern life and poses an acknowledged risk factor for many somatic and psychiatric disorders, which are often associated with chronic low-grade inflammation.(1-8) Many clinical and preclinical studies(6, 9-14) support the hypothesis that stress-associated inflammation is promoted at least in part via development of glucocorticoid (GC) resistance, defined as a state of reduced sensitivity to the anti-inflammatory action of GCs, in certain immune cell subpopulations(6, 11) amongst which myeloid CD11b+ cells seem to play a critical role.(12, 13, 15) Noteworthy, over-shooting local and/ or systemic inflammatory responses(16-22) as well as development of GC resistance(23-25) further promote posttraumatic complications (e.g., septic shock), for patients on intensive care. Therefore, a history of chronic/traumatic psychosocial stress and the subsequent development of GC resistance is a plausible scenario in vulnerable subgroups of physical trauma patients on intensive care. Of note, MDSCs represent immature myeloid cells, are generated in the bone marrow and able to suppress T cell proliferation via generation of nitric oxide (NO) and reactive oxygen species, as well as via depletion of arginine and down-regulation of the T cell receptor complex ζ chain, and have been first described in tumor patients and tumor-bearing mice.(26-28) As MDSCs are also induced during bacterial infections,(29, 30) they seem to provide a cellular link between activation of innate immunity and concomitant suppression of adaptive immunity.
Main findings: In a series of preclinical experiments employing the chronic subordinate colony housing (CSC) paradigm as an acknowledged model for social stress-associated posttraumatic stress disorder (PTSD) in male mice(31, 32) we could show that particularly CD11b+Ly6G+Ly6C+ polymorphonuclear (PMN)-myeloid-derived suppressor cells (MDSCs) play a critical role in psychosocial stress-induced splenic ex vivo functional GC resistance.(33-36) In detail, we showed that CSC accompanied by significant wounding and, thus, a combination of psychosocial and physical trauma, i) enhanced basal and LPS-induced ex vivo cell viability of isolated BM cells, ii) increased the percentage of toll-like receptor (TLR)2-expressing bone marrow (BM) and spleen CD11b+Ly6G+Ly6C- neutrophils, PMN-MDSCs and CD11b+Ly6G-Ly6C+ monocytes/MO-MDSCs, iii) increased the percentage of TLR4-expressing spleen PMN-MDSCs and monocytes/ mononuclear (MO)-MDSCs, iv) enhanced basal and LPS-induced ex vivo cell viability of isolated PMN-MDSC-enriched PBMCs and splenocytes, as well as ex vivo migration activity of neutrophil/PMN-MDSC-enriched WBCs, v) induced ex vivo GC resistance in LPS-stimulated Ly6G+ splenocytes but not Ly6G-depleted total splenocytes or PMN-MDSC-enriched PBMCs, vi) rendered stress-induced Ly6G+ splenocytes to increase cell viability upon LPS stimulation exclusively via the NF-κB pathway.(37) These results support the hypothesis that stress-induced PMN-MDSCs get primed(37) and activated locally in the bone marrow (BM) as determined by toll-like receptor (TLR)2 and TH, but not TLR4, upregulation and increased basal and lipopolysaccharide (LPS)-induced ex vivo cell viability.(33) These primed and activated PMN-MDSCs emigrate into the peripheral circulation and subsequently, if psychosocial stress is accompanied by significant bite wounding, accumulate in the spleen(33). In the spleen, PMN-MDSCs upregulate TLR4 expression, which in concert with PMN-MDSCs-derived catecholamines as a consequence of increased TH expression promotes NF-κB hyperactivation upon LPS-stimulation, thereby exceeding the anti-inflammatory capacities of GCs and resulting in GC resistance.(33) Upregulation of myeloid-derived catecholamines as a consequence of TH upregulation has been shown to promote NF-κB signaling and to augment the acute inflammatory response to acute lung injury.(38, 39)
Main collaborators: Prof. Dr. Jan Tuckermann & Prof. Dr. Maja Vujić Spasić (Ulm University, Ulm, Germany), Prof. Dr. Steffen Stenger (Ulm University Medical Center, Ulm, Germany).
References:
1. Rohleder N (2014): Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 76:181-189.
2. Rohleder N, Marin TJ, Ma R, Miller GE (2009): Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. J Clin Oncol. 27:2909-2915.
3. Langgartner D, Lowry CA, Reber SO (2019): Old Friends, immunoregulation, and stress resilience. Pflügers Archiv - European Journal of Physiology.1-33.
4. Langgartner D, Fuchsl AM, Uschold-Schmidt N, Slattery DA, Reber SO (2015): Chronic subordinate colony housing paradigm: a mouse model to characterize the consequences of insufficient glucocorticoid signaling. Frontiers in psychiatry. 6:18.
5. Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T (2017): Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology. 42:254-270.
6. Miller AH, Raison CL (2016): The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 16:22-34.
7. Gould TD, Georgiou P, Brenner LA, Brundin L, Can A, Courtet P, et al. (2017): Animal models to improve our understanding and treatment of suicidal behavior. Translational psychiatry. 7:e1092.
8. Schultebraucks K, Qian M, Abu-Amara D, Dean K, Laska E, Siegel C, et al. (2021): Pre-deployment risk factors for PTSD in active-duty personnel deployed to Afghanistan: a machine-learning approach for analyzing multivariate predictors. Mol Psychiatry. 26:5011-5022.
9. Bellingrath S, Rohleder N, Kudielka BM (2013): Effort-reward-imbalance in healthy teachers is associated with higher LPS-stimulated production and lower glucocorticoid sensitivity of interleukin-6 in vitro. Biol Psychol. 92:403-409.
10. Raison CL, Capuron L, Miller AH (2006): Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 27:24-31.
11. Raison CL, Miller AH (2003): When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 160:1554-1565.
12. Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF (2001): Social stress induces glucocorticoid resistance in macrophages. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 280:R1799-R1805.
13. Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF (2002): Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J Neuroimmunol. 124:54-61.
14. Engler H, Engler A, Bailey MT, Sheridan JF (2005): Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J Neuroimmunol. 163:110-119.
15. Engler H, Bailey MT, Engler A, Sheridan JF (2004): Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 148:106-115.
16. Perl M, Hohmann C, Denk S, Kellermann P, Lu D, Braumuller S, et al. (2012): Role of activated neutrophils in chest trauma-induced septic acute lung injury. Shock. 38:98-106.
17. Seitz DH, Niesler U, Palmer A, Sulger M, Braumuller ST, Perl M, et al. (2010): Blunt chest trauma induces mediator-dependent monocyte migration to the lung. Crit Care Med. 38:1852-1859.
18. Knoferl MW, Liener UC, Seitz DH, Perl M, Bruckner UB, Kinzl L, et al. (2003): Cardiopulmonary, histological, and inflammatory alterations after lung contusion in a novel mouse model of blunt chest trauma. Shock. 19:519-525.
19. Perl M, Gebhard F, Knoferl MW, Bachem M, Gross HJ, Kinzl L, et al. (2003): The pattern of preformed cytokines in tissues frequently affected by blunt trauma. Shock. 19:299-304.
20. Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, et al. (1998): Outcome after hemorrhagic shock in trauma patients. J Trauma. 45:545-549.
21. Kauvar DS, Wade CE (2005): The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 9 Suppl 5:S1-9.
22. Angele MK, Schneider CP, Chaudry IH (2008): Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 12:218.
23. Rothwell PM, Udwadia ZF, Lawler PG (1991): Cortisol response to corticotropin and survival in septic shock. Lancet. 337:582-583.
24. Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E (2000): A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 283:1038-1045.
25. Molijn GJ, Spek JJ, van Uffelen JC, de Jong FH, Brinkmann AO, Bruining HA, et al. (1995): Differential adaptation of glucocorticoid sensitivity of peripheral blood mononuclear leukocytes in patients with sepsis or septic shock. J Clin Endocrinol Metab. 80:1799-1803.
26. Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. (2010): Myeloid-derived suppressor cell heterogeneity and subset definition. Current opinion in immunology. 22:238-244.
27. Gabrilovich DI, Nagaraj S (2009): Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews immunology. 9:162-174.
28. Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI (2010): Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 184:3106-3116.
29. Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, et al. (2007): MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 204:1463-1474.
30. Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, et al. (2011): Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 186:195-202.
31. Reber SO, Langgartner D, Foertsch S, Postolache TT, Brenner LA, Guendel H, et al. (2016): Chronic subordinate colony housing paradigm: A mouse model for mechanisms of PTSD vulnerability, targeted prevention, and treatment—2016 Curt Richter Award Paper. Psychoneuroendocrinology. 74:221-230.
32. Reber S, Birkeneder L, Veenema A, Obermeier F, Falk W, Straub R, et al. (2007): Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 148:670-682.
33. Kempter E, Amoroso M, Kupfer S, Lupu L, Kustermann M, Scheurer J, et al. (2023): The PMN-MDSC – A key player in glucocorticoid resistance following combined physical and psychosocial trauma. Brain, Behavior, and Immunity. 108:148-161.
34. Foertsch S, Reber SO (2020): The role of physical trauma in social stress-induced immune activation. Neurosci Biobehav Rev. 113:169-178.
35. Foertsch S, Langgartner D, Reber SO (2020): Abdominal surgery prior to chronic psychosocial stress promotes spleen cell (re)activity and glucocorticoid resistance. Scientific Reports. 10:6917.
36. Foertsch S, Füchsl AM, Faller SD, Hölzer H, Langgartner D, Messmann J, et al. (2017): Splenic glucocorticoid resistance following psychosocial stress requires physical injury. Scientific Reports. 7:15730.
37. Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF (2012): Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain, Behavior, and Immunity. 26:1150-1159.
38. Flierl MA, Rittirsch D, Nadeau BA, Sarma JV, Day DE, Lentsch AB, et al. (2009): Upregulation of Phagocyte-Derived Catecholamines Augments the Acute Inflammatory Response. PLoS ONE. 4:e4414.
39. Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, et al. (2007): Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 449:721-725.
P3
Stress-induced bone defects
It is the main aim of this project to investigate in preclinical and clinical studies the cellular, molecular and microbiome-related mechanisms underlying the sex-specific effects of chronic psychosocial stress during early life (=early life adversity, ELA), adulthood (CAS) and a combination of both (ELA&CAS) on bone homeostasis and regeneration. Note, with respect to ELA we study the effects of stressors occurring during the prenatal and postnatal phase on both male and female offspring and their mothers.
Background: Chronic psychosocial stress during adulthood(1, 2) as well as early life adversity (ELA)(3-7) are acknowledged risk factors for several psychosomatic disorders, including posttraumatic stress disorder (PTSD) and major depression (MD). Both diseases display a high prevalence in western countries, are strongly comorbid with various somatic pathologies(8, 9) and have been associated with osteoporosis and increased bone fracture risk in a number of studies.(8-12). However, while there is strong evidence for an increased risk for low bone mass and fragility fractures in depressed patients being mediated by increased glucocorticoid (GC) concentrations,(13, 14) findings in PTSD patients are less consistent. For example, multivariable analyses controlling for depression in PTSD subjects failed to demonstrate a link between PTSD and osteoporosis,(15) whereas earlier studies stated a significant association between these conditions.(12) Furthermore, PTSD may influence long-bone growth: children subjected to repeated mental traumatization during childhood were of a significantly shorter stature.(16) In summary, these clinical studies implicate different effects of stress-induced depression and PTSD on bone turnover.
Main findings: In contrast to mouse models for depression, employing the chronic subordinate colony housing (CSC) paradigm as an acknowledged model for social stress-associated PTSD in male mice(17, 18) we showed that mental traumatization in adolescent mice negatively impacts cartilage-to-bone transition during endochondral ossification in the epiphyseal growth plate, the main site of longitudinal growth of the long bones, while appositional bone growth seems to be undisturbed.(19) In detail, CSC mice show reduced tibia and femur lengths, mineral deposition at the growth plate and Runt-related transcription factor 2 (Runx2) expression in hypertrophic chondrocytes in the growth plate, while growth plate and trabecular thickness as well as bone mineral density (BMD) were increased in CSC compared to single-housed control (SHC) mice.(19) An enhanced tyrosine hydroxylase (TH) expression, which is the rate limiting enzyme in catecholamine (CA) synthesis,(20) in bone marrow (BM) cells located at the growth plates of CSC mice suggests that local CA signalling is involved in the negative CSC effects on bone metabolism.(19) Of note in this context, norepinephrine (NE) release by sympathetic nerve fibers during chronic variable stress signals bone marrow niche cells to decrease CXCL12 levels through the β3-adrenoreceptor, resulting in increased hematopoietic stem cell proliferation and release of neutrophils and inflammatory monocytes.(21) In a follow up study we extended these findings by revealing that CSC mice undergoing standardized femur fracture show a delayed bone healing, again accompanied by a compromised cartilage-to-bone transition. Furthermore, CSC mice were characterized by a misbalanced inflammatory response in the fracture hematoma.(22) The latter was indicated by increased numbers of TH expressing neutrophils, and both delayed fracture healing and hematoma invasion of TH expressing neutrophils were prevented in CSC mice by injection with an unspecific β-adrenoceptor blocker prior to fracture surgery.(22) In a recent study(23) we provide evidence supporting the conclusion that while impaired mental health and stress in general promotes BM myelopoiesis, TH expression and, consequently, the capacity to produce/ secrete CAs is specifically facilitated in neutrophils. Neutrophil-derived CAs locally in the BM activate α (in vitro data)/β2 (in vitro and in vivo data)-ARs and dopaminergic receptors (DRs, in vitro data) on chondrocytes and, consequently, compromise their transdifferentiation into osteoblasts and, thus, bone metabolism. Neutrophil-derived CAs in an autocrine manner further promote their own BM emigration and, in case of a fracture, facilitate their own immigration into the fracture hematoma, likely in a paracrine manner by increasing CXCL1 release from hematoma mast cells and macrophages which are two main CXCL1 producing cell types.(24) In the fracture hematoma, neutrophil-derived CAs again activate α/β2-ARs and DRs on chondrocytes and, consequently, compromise their transdifferentiation into osteoblasts and, thus, adequate bone repair. According to our clinical data,(23) indicating an increased TH expression in fracture hematomas of patients with an increased mental stress load, which is further accompanied by a compromised fracture healing and/or increased pain sensitivity, our preclinical data seem to be of high translational value, suggesting strategies to block immigration of TH positive myeloid cells/ neutrophils into the fracture hematoma or their local release of CAs to represent promising future strategies to facilitate fracture healing in patients who are at risk for psychosomatic disorders.
Main collaborators: Prof. Dr. Melanie Haffner-Luntzer & Prof. Dr. Anita Ignatius (Ulm University, Ulm, Germany), Prof. Dr. Florian Gebhard & Prof. Dr. Konrad Schütze (Ulm University Medical Center, Ulm, Germany).
References:
1. Yehuda R, Seckl J (2011): Minireview: Stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology. 152:4496-4503.
2. Gold PW, Goodwin FK, Chrousos GP (1988): Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (1). The New England journal of medicine. 319:348-353.
3. Kuzminskaite E, Vinkers CH, Elzinga BM, Wardenaar KJ, Giltay EJ, Penninx BWJH (2020): Childhood Trauma and Dysregulation of Multiple Biological Stress Systems in Adulthood: Results from the Netherlands Study of Depression and Anxiety. Psychoneuroendocrinology.104835.
4. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. (2019): REPRINT OF: Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 56:774-786.
5. Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. (2017): The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health. 2:e356-e366.
6. Maercker A, Augsburger M (2019): Die posttraumatische Belastungsstörung. Traumafolgestörungen: Springer, pp 13-45.
7. Steil R, Rosner R (2009): Posttraumatische Belastungsstörung bei Kindern und Jugendlichen. In: Maercker A, editor. Posttraumatische Belastungsstörungen. Berlin, Heidelberg: Springer Berlin Heidelberg, pp 321-343.
8. Gebara MA, Shea ML, Lipsey KL, Teitelbaum SL, Civitelli R, Muller DJ, et al. (2014): Depression, antidepressants, and bone health in older adults: a systematic review. J Am Geriatr Soc. 62:1434-1441.
9. Glaesmer H, Kaiser M, Braehler E, Freyberger HJ, Kuwert P (2012): Posttraumatic stress disorder and its comorbidity with depression and somatisation in the elderly - a German community-based study. Aging Ment Health. 16:403-412.
10. Calarge CA, Butcher BD, Burns TL, Coryell WH, Schlechte JA, Zemel BS (2014): Major depressive disorder and bone mass in adolescents and young adults. J Bone Miner Res. 29:2230-2237.
11. Zong Y, Tang Y, Xue Y, Ding H, Li Z, He D, et al. (2016): Depression is associated with increased incidence of osteoporotic thoracolumbar fracture in postmenopausal women: a prospective study. Eur Spine J. 25:3418-3423.
12. Glaesmer H, Brahler E, Gundel H, Riedel-Heller SG (2011): The association of traumatic experiences and posttraumatic stress disorder with physical morbidity in old age: a German population-based study. Psychosom Med. 73:401-406.
13. Cizza G, Ravn P, Chrousos GP, Gold PW (2001): Depression: a major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab. 12:198-203.
14. Cizza G, Primma S, Coyle M, Gourgiotis L, Csako G (2010): Depression and Osteoporosis: A Research Synthesis with Meta-Analysis. Horm Metab Res. 42:467-482.
15. Tsai J, Shen J (2017): Exploring the Link Between Posttraumatic Stress Disorder and inflammation-Related Medical Conditions: An Epidemiological Examination. Psychiatr Q.
16. Batty GD, Shipley MJ, Gunnell D, Huxley R, Kivimaki M, Woodward M, et al. (2009): Height, wealth, and health: an overview with new data from three longitudinal studies. Econ Hum Biol. 7:137-152.
17. Reber SO, Langgartner D, Foertsch S, Postolache TT, Brenner LA, Guendel H, et al. (2016): Chronic subordinate colony housing paradigm: A mouse model for mechanisms of PTSD vulnerability, targeted prevention, and treatment—2016 Curt Richter Award Paper. Psychoneuroendocrinology. 74:221-230.
18. Reber S, Birkeneder L, Veenema A, Obermeier F, Falk W, Straub R, et al. (2007): Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 148:670-682.
19. Foertsch S, Haffner-Luntzer M, Kroner J, Gross F, Kaiser K, Erber M, et al. (2017): Chronic psychosocial stress disturbs long-bone growth in adolescent mice. Dis Model Mech. 10:1399-1409.
20. Molinoff PB, Axelrod J (1971): Biochemistry of catecholamines. Annu Rev Biochem. 40:465-500.
21. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. (2014): Chronic variable stress activates hematopoietic stem cells. Nature medicine. 20:754.
22. Haffner-Luntzer M, Foertsch S, Fischer V, Prystaz K, Tschaffon M, Mödinger Y, et al. (2019): Chronic psychosocial stress compromises the immune response and endochondral ossification during bone fracture healing via β-AR signaling. Proceedings of the National Academy of Sciences.201819218.
23. Tschaffon-Müller MEA, Kempter E, Steppe L, Kupfer S, Kuhn MR, Gebhard F, et al. (2023): Neutrophil-derived catecholamines mediate negative stress effects on bone. Nat Commun. 14:3262.
24. De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. (2013): Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 121:4930-4937.
P4
Understanding the mechanisms underlying the increased prevalence of mental and physical stress-associated disorders in urban vs. rural areas.
It is the main aim of this clinical project to investigate the cellular, molecular and microbiome-related mechanisms underlying the increased prevalence of stress-associated mental as well as physical disorders in individuals raised in an urban (URBANs) versus rural (RURALs) environment.
Background: Urbanization is on the rise,(1) and stress-associated somatic and mental disorders are more prevalent in urban vs. rural areas.(2-4) Many of these disorders are accompanied by an over-reactive immune system and chronic low-grade inflammation,(5, 6) and prospective human and mechanistic animal studies strengthen the idea that an exaggerated immune (re)activity plays a causal role in their pathogenesis.(5, 7-9) Deficits in immunoregulation are thought to be in part dependent on reduced exposure, especially during early life,(10, 11) to microorganisms with which mammals co-evolved.(12)These “Old Friends” needed to be tolerated, as they were either part of host physiology (human microbiota), harmless but inevitably contaminating air, food and water (environmental microbiota), or causing severe tissue damage when attacked by the host immune system (helminthic parasites).(12) However, contact with these microorganisms that play a crucial role in setting up regulatory immune pathways is slowly but progressively diminishing in high-income countries, particularly in the concrete landscapes of urban areas.(11, 13) Noteworthy, the decline in biodiversity is currently facilitated to unprecedented levels due to dramatic changes in global climate, excessive levels of environmental pollution as well as recent COVID-19-related restrictions. Besides the declining availability of health promoting green space rich in biodiversity,(14, 15) another critical factor contributing to the diminishing contact with “Old Friends”, particularly in urban areas, seems to be the lack of regular contact with animals.(16-19) Supporting this hypothesis, dog ownership has been shown to increase microbial diversity and relative abundances of dog-associated bacterial taxa across multiple locations within the home.(20, 21)
Main findings: In a recent study (Urban vs. Rural Stress Study, URSS) we demonstrated that systemic immune activation in response to a standardized laboratory social stressor, namely the Trier Social Stress Test (TSST), is increased and prolonged in healthy male URBANs raised in the absence of daily contact with pets, relative to healthy male RURALs raised in the presence of farm animals, even though the inflammatory stress response triggering HPA axis and SNS activation were more pronounced in the latter.(22) In detail, in response to the TSST, URBANs raised in the absence of pets showed a more pronounced increase in the number of peripheral blood mononuclear cells (PBMCs) and plasma interleukin (IL)-6 concentrations compared with RURALs raised in the presence of farm animals. Moreover, ex vivo cultured PBMCs from URBANs raised in the presence of pets secreted more IL-6 in response to the T cell-specific mitogen concanavalin A (ConA) than respective PBMCs from RURALs raised in the presence of farm animals. In turn, anti-inflammatory IL-10 secretion was suppressed following TSST in URBANs raised in the absence of pets, suggesting immunoregulatory deficits in urban participants following social stress. Importantly, URBANs reporting absolutely no pet contact differed in their salivary microbiome composition from all other URBANs and RURALs reporting regular or at least occasional contact to farm animals or pets belonging to others households, and displayed a significantly higher TSST-induced immune activation compared to URBANs reporting at least occasional contact to pets belonging to others,(23) suggesting that the complete absence of any pet contact plays a critical role in mediating the negative consequences of urban upbringing. The other way round, having a pet may mitigate some of the decreases in exposures to diverse microbial environments and the associated immunoregulatory deficits in those living in modern urban environments and, therefore, represent a primary prevention strategy for chronic low-grade inflammation and development of any kind of stress-associated disorder linked to an (over)activated immune system. In line with this hypothesis we showed in a follow-up study (Effects of Pets on Social Stress, EPSS) that adult healthy male URBANs raised in the absence (noPETs) vs. presence (PETs) of household pets were characterized by deficits in their immunoregulatory and intestinal barrier function, which under basal conditions did not translate into a chronic low-grade inflammatory state.(24) This was different under acute psychosocial stress conditions. Exposure to the TSST resulted in a facilitated mobilization of particularly neutrophil granulocytes in noPETs vs. PETs, accompanied by an enhanced pro- and compromised anti-inflammatory systemic stress response.(24) Together, the presence of pets during urban upbringing seems to reduce the risk for developing stress-associated disorders later in life (i.e., primary prevention) by facilitating immunoregulatory and barrier functions, in turn preventing an overshooting immune activation in response to acute stressors and chronic low-grade inflammation in response to repeated/chronic stressors.
Main collaborators: Prof. Dr. Christopher Lowry (University of Colorado, Boulder, USA), Prof. Dr. Harald Gündel, Dr. Katja Weimer, Dr. Marc Jarczok, Prof. Dr. Hans Kestler, Dr. Alexander Groß, Prof. Dr. Markus Huber-Lang (Ulm University or Ulm University Medical Center, Ulm, Germany), Prof. Dr, Andreas Meyer-Lindenberg, Prof. Dr. Heike Tost (Central Institute of Mental Health, Mannheim, Germany), Prof. Nicolas Rohleder (Friedrich-Alexander-Universität, Erlangen, Germany), Prof. Dr. Graham Rook (University College London (UCL), London, United Kingdom)
References:
1. United Nations DoEaSA, Population Division (2014): World Urbanization Prospects: The 2014 Revision, Highlights (ST/ESA/SER.A/352).
2. Peen J, Schoevers RA, Beekman AT, Dekker J (2010): The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand. 121:84-93.
3. Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. (2001): Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. The Lancet. 358:1129-1133.
4. Langgartner D, Lowry CA, Reber SO (2019): Old Friends, immunoregulation, and stress resilience. Pflügers Archiv - European Journal of Physiology.1-33.
5. Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. (2006): Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 163:1630-1633.
6. Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, et al. (2013): Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 13:40.
7. Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. (2014): Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 111:16136-16141.
8. Kivimaki M, Shipley MJ, Batty GD, Hamer M, Akbaraly TN, Kumari M, et al. (2014): Long-term inflammation increases risk of common mental disorder: a cohort study. Mol Psychiatry. 19:149-150.
9. Rohleder N (2014): Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 76:181-189.
10. Rook GA, Lowry CA, Raison CL (2013): Microbial 'Old Friends', immunoregulation and stress resilience. Evolution, medicine, and public health. 2013:46-64.
11. Rook GA, Raison CL, Lowry CA (2013): Childhood microbial experience, immunoregulation, inflammation and adult susceptibility to psychosocial stressors and depression in rich and poor countries. Evolution, medicine, and public health. 2013:14-17.
12. Rook GA (2013): Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci U S A. 110:18360-18367.
13. Martínez I, Stegen James C, Maldonado-Gómez Maria X, Eren AM, Siba Peter M, Greenhill Andrew R, et al. (2015): The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Reports. 11:527-538.
14. Engemann K, Pedersen CB, Arge L, Tsirogiannis C, Mortensen PB, Svenning J-C (2019): Residential green space in childhood is associated with lower risk of psychiatric disorders from adolescence into adulthood. Proceedings of the National Academy of Sciences.201807504.
15. Yang B-Y, Zeng X-W, Markevych I, Bloom MS, Heinrich J, Knibbs LD, et al. (2019): Association Between Greenness Surrounding Schools and Kindergartens and Attention-Deficit/Hyperactivity Disorder in Children in China. JAMA Network Open. 2:e1917862-e1917862.
16. Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. (2016): Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med. 375:411-421.
17. Fall T, Lundholm C, Ortqvist AK, Fall K, Fang F, Hedhammar A, et al. (2015): Early exposure to dogs and farm animals and the risk of childhood asthma. JAMA Pediatr. 169:e153219.
18. Mubanga M, Byberg L, Nowak C, Egenvall A, Magnusson PK, Ingelsson E, et al. (2017): Dog ownership and the risk of cardiovascular disease and death - a nationwide cohort study. Sci Rep. 7:15821.
19. Okabe H, Hashimoto K, Yamada M, Ono T, Yaginuma K, Kume Y, et al. (2023): Associations between fetal or infancy pet exposure and food allergies: The Japan Environment and Children’s Study. PLoS ONE. 18:e0282725.
20. Dunn RR, Fierer N, Henley JB, Leff JW, Menninger HL (2013): Home life: factors structuring the bacterial diversity found within and between homes. PLoS ONE. 8:e64133.
21. Mäki JM, Kirjavainen PV, Täubel M, Piippo-Savolainen E, Backman K, Hyvärinen A, et al. (2021): Associations between dog keeping and indoor dust microbiota. Sci Rep. 11:5341.
22. Böbel TS, Hackl SB, Langgartner D, Jarczok MN, Rohleder N, Rook GA, et al. (2018): Less immune activation following social stress in rural vs. urban participants raised with regular or no animal contact, respectively. Proc Natl Acad Sci U S A. 115:5259-5264.
23. Langgartner D, Zambrano CA, Heinze JD, Stamper CE, Böbel TS, Hackl SB, et al. (2020): Association of the Salivary Microbiome With Animal Contact During Early Life and Stress-Induced Immune Activation in Healthy Participants. Frontiers in Psychiatry. 11.
24. Langgartner D, Weimer K, Brunner-Weisser J, Winkler R, Mannes M, Huber-Lang M, et al. (under review): PAWsitive impact: How pet contact during urban childhood ameliorates adult inflammatory stress responses.
For NEWS follow us on X: @MolPsySoUlm
News
- Prof. Reber as guest at ARTE Saloon Live-Talk: "Können Mikroben alles?"
- The Reber Lab on TV - Doc Fischer at SWR: "Stress & Knochen"
- 2023 GEBIN Annual Meeting - the Reber MPS lab as local organizer
- Prof. Reber on YouTube - Blog "Immunsignature" by Dr. med. Keferstein (Mojo Institute for Regenerative Medicince)
- The Reber Lab on TV - Wissen hoch 2 at 3SAt: "Schwelbrand im Körper"