(Epi) Genetics

During life, our organism is exposed to many different risk factors which induce epigenetic changes that finally contribute to the regulation of gene expression. An insight into the cumulative effect of DNA and histone modifications provide the chromatin landscape. Furthermore, chromatin plays a critical role in the regulation of gene expression, as only accessible genomic regions allow the transcriptional machinery to interact with the DNA.
For several neurodegenerative diseases epigenetic mechanisms have been proposed to be involved.
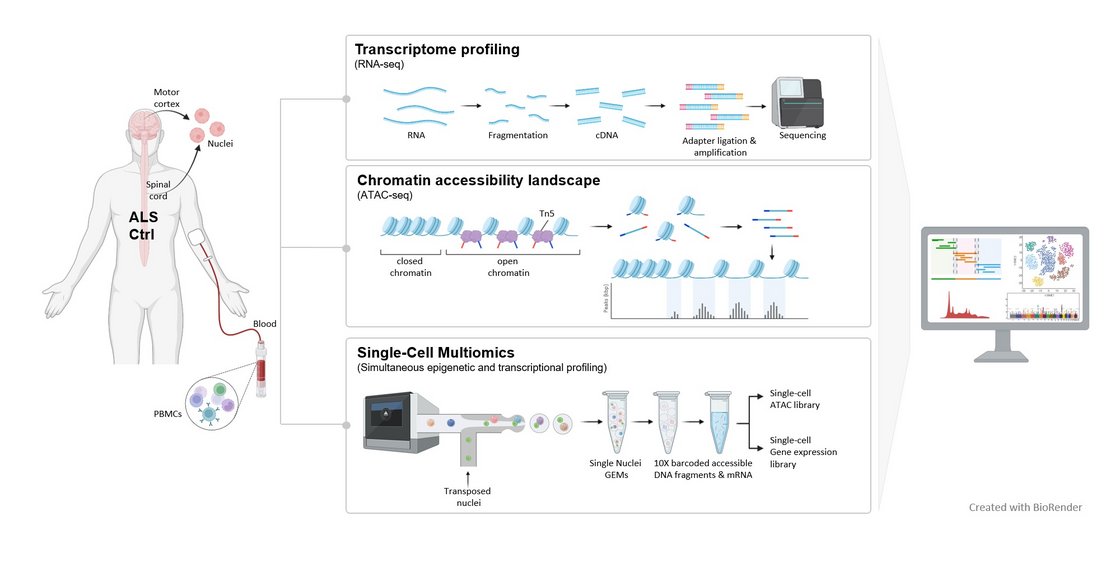
To investigate a mechanistic link between chromatin accessibility and ALS disease pathogenesis, we use a multi-omic approach combining an assay for transposase accessible chromatin using sequencing (ATAC-seq) and RNA-seq at bulk and single-cell level in peripheral blood mononuclear cells (PBMCs) from sporadic ALS patients and healthy controls, and integrate these results by thorough data science analysis.
We were able to show that a genome-wide signature of ALS (‘epiChromALS’) can be found in the chromatin accessibility of PBMCs from sporadic ALS patients. Functional enrichment analysis of these differentially accessible peaks revealed an association with neuronal functions and neuronal differentiation in the less accessible genomic regions, suggesting that neurodevelopmental processes are epigenetically impaired in ALS. These common epigenetic marks were associated with neuronal terms, suggesting that they are systemic.
To further corroborate our findings of a peripherally detectable epigenetic chromatin accessibility signature, we set up a larger scaled study where we investigate the chromatin accessibility in combination with gene expression profiling in single-nuclei of post-mortem tissue from the areas most affected in ALS, such as the motor cortex and spinal cord. Dysregulated epigenetic patterns in different cell types and tissues and their effects on gene expression programs are still underexplored, although they provide new insights into cell-specific and systemic alterations. Especially the understanding of systemic changes may open new doors for the development of new therapies focusing on the design of epigenetic drugs.
People


Dr. Veselin Grozdanov
M.Sc. Biochemistry
Phone: +49-731-500-63152
Fax: +49-731-500-63050
Mail: veselin.grozdanov@uni-ulm.de