Head

Prof. Dr. Pamela Fischer-Posovszky
Ulm University Medical Center
Department of Pediatrics and Adolescent Medicine
Eythstr. 24 (Research Lab, House 16)
89075 Ulm, Germany
phone: +49-731-500 57415
fax: +49-731-500 57412
e-mail: pamela.fischer@uniklinik-ulm.de
Homepage Profile Prof. Dr. Fischer-Posovszky

| Name | First Name | Function | Phone | E-mail |
|---|---|---|---|---|
| Prof. Dr. Fischer-Posovszky | Pamela | Head | +49-731-500 57415 | pamela.fischer@uniklinik-ulm.de |
| Halbgebauer | Daniel | PhD Student | +49-731-500 57261 | daniel.halbgebauer@uniklinik-ulm.de |
| Killian | Alexandra | TA | +49-731-500 57421 | alexandra.killian@uniklinik-ulm.de |
| Pula | Taner | PhD Student | +49-731-500 57261 | taner.pula@uniklinik-ulm.de |
| Dr. rer. nat. Roos | Julian | Post-Doc | +49-731-500 57255 | julian.roos@uniklinik-ulm.de |
| Schlichtig | Ferdinand | TA | +49-731-500 57086 | ferdinand.schlichtig@uniklinik-ulm.de |
| Dr. rer. nat. Tews | Daniel | Post-Doc | +49-731-500 57418 | daniel.tews@uniklinik-ulm.de |
| Wu | Hang | MD Student | +49-731-500 57282 | hang.wu@uniklinik-ulm.de |
| Dr. med. Zinngrebe | Julia | Post-Doc | +49-731-500 57086 | julia.zinngrebe@uniklinik-ulm.de |
Research Profile
Obesity is a worldwide epidemic. The excessive accumulation of adipose tissue leads to the development of severe comorbidities such as insulin resistance, type 2 diabetes mellitus, hepatic steatosis, cardiovascular diseases including hypertension and atherosclerosis, and an increased risk of developing certain types of cancer. Conventional therapy concepts involving e.g. diet, physical exercise, or behavior therapy often fail. Thus, there is an urgent need to develop innovative pharmacological treatment strategies. In our group we aim to understand the physiology and pathophysiology of adipose tissue.

Adipose tissue is a dynamic organ with ~10% of fat cells being renewed annually. Our group investigates the role of death receptors in this remodeling process.
We found out that preadipocytes and adipocytes express death receptors, among them CD95, TNF receptors, and TRAIL receptors. Interestingly, both cell types are protected from apoptosis induced by their respective ligands. Apoptosis of adipocytes can only be induced under certain conditions, e.g. by inhibition of protein biosynthesis (Fischer-Posovszky et al., Endocrinology 2004).
In our current projects we study non-apoptotic function of death receptors in adipose tissue. We have elucidated that TRAIL stimulates the proliferation of preadipocytes and inhibits their adipogenic differentiation (Funcke et al., FASEB J 2015; Zoller et al., Cell Death Dis 2016).
Zoller V, Funcke JB, Roos J, Dahlhaus M, Abd El Hay M, Holzmann K, Marienfeld R, Kietzmann T, Debatin KM, Wabitsch M, Fischer-Posovszky P: TRAIL (TNF-related apoptosis-inducing ligand) induces an inflammatory response in human adipocytes. Sci Rep. 2017 Jul 18;7(1):5691. doi: 10.1038/s41598-017-05932-7.
Zoller V, Funcke JB, Keuper M, Abd El Hay M, Debatin KM, Wabitsch M, Fischer-Posovszky P: TRAIL (TNF-related apoptosis-inducing ligand) inhibits human adipocyte differentiation via caspase-mediated downregulation of adipogenic transcription factors. Cell Death Dis. 2016 Oct 13;7(10):e2412. doi: 10.1038/cddis.2016.286.
Funcke JB, Zoller V, El Hay MA, Debatin KM, Wabitsch M, Fischer-Posovszky P: TNF-related apoptosis-inducing ligand promotes human preadipocyte proliferation via ERK1/2 activation. FASEB J. 2015 Jul;29(7):3065-75. doi: 10.1096/fj.14-267278.
Keuper M, Wernstedt Asterholm I, Scherer PE, Westhoff MA, Möller P, Debatin KM, Strauss G, Wabitsch M, Fischer-Posovszky P: TRAIL (TNF-related apoptosis-inducing ligand) regulates adipocyte metabolism by caspase-mediated cleavage of PPARgamma. Cell Death Dis. 2013 Jan 24;4:e474. doi: 10.1038/cddis.2012.212.
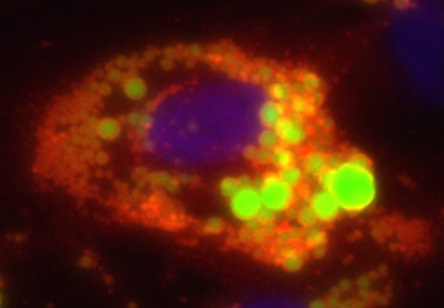
MicroRNAs (miRNAs) are small, 18-25 nucleotide long, non-coding RNA molecules. They are central regulators of gene expression and influence a variety of biological processes including cellular differentiation and metabolism.
Obese adipose tissue is characterized by pathological alterations such as hypertrophy of adipocytes, inflammation, hypoxia, and fibrosis. We showed that miRNAs are differentially regulated by inflammatory stimuli in adipocytes (Roos et al., Sci Rep 2016). We now aim at identifying the function of specific miRNAs. miR-146a for example was identified as a negative regulator of the inflammatory response in adipocytes (Roos et al., Sci Rep 2016).
miRNAs can be released to the circulation. We also seek to find out if these small RNA molecules might constitute novel biomarkers of adipose tissue health.
Mysore R, Ortega FJ, Latorre J, Ahonen M, Savolainen-Peltonen H, Fischer-Posovszky P, Wabitsch M, Olkkonen VM, Fernández-Real JM, Haridas PAN: MicroRNA-221-3p regulates angiopoietin-like 8 (ANGPTL8) expression in adipocytes. J Clin Endocrinol Metab. 2017 Nov 1;102(11):4001-4012. doi: 10.1210/jc.2017-00453.
Roos J, Enlund E, Funcke JB, Tews D, Holzmann K, Debatin KM, Wabitsch M, Fischer-Posovszky P: miR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci Rep. 2016 Dec 6;6:38339. doi: 10.1038/srep38339
Fischer-Posovszky P, Roos J, Kotnik P, Battelino T, Inzaghi E, Nobili V, Cianfarani S, Wabitsch M: Functional significance and predictive value of microRNAs in pediatric obesity: tiny molecules with huge impact? Horm Res Paediatr. 2016;86(1):3-10. doi: 10.1159/000444677.
Mysore R, Zhou Y, Sädevirta S, Savolainen-Peltonen H, Nidhina Haridas PA, Soronen J, Leivonen M, Sarin AP, Fischer-Posovszky P, Wabitsch M, Yki-Järvinen H, Olkkonen VM: MicroRNA-192* impairs adipocyte triglyceride storage. Biochim Biophys Acta. 2016 Apr;1861(4):342-51. doi: 10.1016/j.bbalip.2015.12.019.

The discovery of active brown adipose tissue in adult humans and its negative association with fat mass and body weight gave rise to the idea, that this special tissue could be utilized for the treatment of obesity and metabolic disease.
Brown adipocytes are characterized by the expression of uncoupling protein-1 (UCP1). This mitochondrial protein is capable of uncoupling cellular respiration from ATP synthesis. The proton gradient, which is built up by the electron transport chain is not used for the production of ATP. Instead, energy is dissipated as heat. UCP1 is activated by cold or β-adrenergic agents, which stimulate lipolysis and result in the metabolism of free fatty acids. Therefore, brown adipocytes can consume stored energy. White adipocytes do not express UCP1 and are thus not capable of thermogenesis. They are responsible for the storage of excess energy, which can be mobilized as needed. A third, intermediate phenotype of fat cells was named beige adipocyte. Beige adipocytes express UCP1 and are thermogenic. They can form within white adipose tissue depots, e.g. upon prolonged cold exposure, in a process called “browning”.
Our laboratory investigates white and beige/brown adipose tissue in humans. From surgical operations in the neck region we collected paired samples of subcutaneous, white adipose tissue and brown adipose tissue from the deep neck region (Tews et al., Mol Cell Endocrinol 2014). Progenitor cells isolated from both depots showed a distinct gene expression profile. We currently study whether the differentially expressed genes play a role in white or brown adipogenesis.
Tews D, Fromme T, Keuper M, Hofmann SM, Debatin KM, Klingenspor M, Wabitsch M, Fischer-Posovszky P: Teneurin-2 (TENM2) deficiency induces UCP1 expression in differentiating human fat cells. Mol Cell Endocrinol. 2017 Mar 5;443:106-113. doi: 10.1016/j.mce.2017.01.015.
Tews D, Schwar V, Scheithauer M, Weber T, Fromme T, Klingenspor M, Barth TF, Möller P, Holzmann K, Debatin KM, Fischer-Posovszky P, Wabitsch M. Comparative gene array analysis of progenitor cells from human paired deep neck and subcutaneous adipose tissue. Mol Cell Endocrinol. 2014 Sep;395(1-2):41-50. doi: 10.1016/j.mce.2014.07.011.
Tews D, Fischer-Posovszky P, Fromme T, Klingenspor M, Fischer J, Rüther U, Marienfeld R, Barth TF, Möller P, Debatin KM, Wabitsch M: FTO deficiency induces UCP-1 expression and mitochondrial uncoupling in adipocytes. Endocrinology. 2013 Sep;154(9):3141-51. doi: 10.1210/en.2012-1873.
The hormone leptin is mainly produced by adipocytes to signal the energy state of the body and exerts its functions as a satiety hormone in the brain. Congenital leptin deficiency is caused by mutations in the leptin gene resulting in defects in protein expression and/or secretion. Affected patients suffer from hyperphagia and severe, early-onset obesity. The disease is usually diagnosed by the absence of leptin in the circulation and confirmed by sequencing the leptin gene.
Together with physicians at the Division of Pediatric Endocrinology and Diabetes (Head: Prof. Dr. Martin Wabitsch) and other collaborators, we recently characterized a new form of leptin deficiency – functional leptin deficiency (Wabitsch et al., New Engl J Med 2015). Affected patients have high circulating leptin levels, but the mutated hormone is biologically inactive and therefore not capable of delivering a satiety signal to the brain. The disease can be treated by daily injections of human recombinant leptin leading to a normalization of eating behavior and rapid weight loss (Wabitsch et al., New Engl J Med 2015; Wabitsch et al., J Clin Endocrinol Metab 2015).
Our laboratory investigates the biological functions of leptin and aims to better understand the clinical picture of congenital leptin deficiency.
Nunziata A, Borck G, Funcke JB, Kohlsdorf K, Brandt S, Hinney A, Moepps B, Gierschik P, Debatin KM, Fischer-Posovszky P, Wabitsch M: Estimated prevalence of potentially damaging variants in the leptin gene. Mol Cell Pediatr. 2017 Nov 3;4(1):10. doi: 10.1186/s40348-017-0074-x.
Wabitsch M, Pridzun L, Ranke M, von Schnurbein J, Moss A, Brandt S, Kohlsdorf K, Moepps B, Schaab M, Funcke JB, Gierschik P, Fischer-Posovszky P, Flehmig B, Kratzsch J: Measurement of immunofunctional leptin to detect and monitor patients with functional leptin deficiency. Eur J Endocrinol. 2017 Mar;176(3):315-322. doi: 10.1530/EJE-16-0821.
Wabitsch M, Funcke JB, von Schnurbein J, Denzer F, Lahr G, Mazen I, El-Gammal M, Denzer C, Moss A, Debatin KM, Gierschik P, Mistry V, Keogh JM, Farooqi IS, Moepps B, Fischer-Posovszky P: Severe early-onset obesity due to bioinactive leptin caused by a p.N103K mutation in the leptin gene. J Clin Endocrinol Metab. 2015 Sep;100(9):3227-30. doi: 10.1210/jc.2015-2263.
Fischer-Posovszky P, Funcke JB, Wabitsch M: Biologically inactive leptin and early-onset extreme obesity. N Engl J Med. 2015 Mar 26;372(13):1266-7. doi: 10.1056/NEJMc1501146.
Wabitsch M, Funcke JB, Lennerz B, Kuhnle-Krahl U, Lahr G, Debatin KM, Vatter P, Gierschik P, Moepps B, Fischer-Posovszky P: Biologically inactive leptin and early-onset extreme obesity. N Engl J Med. 2015 Jan 1;372(1):48-54. doi: 10.1056/NEJMoa1406653.
Fischer-Posovszky P, von Schnurbein J, Moepps B, Lahr G, Strauss G, Barth TF, Kassubek J, Mühleder H, Möller P, Debatin KM, Gierschik P, Wabitsch M: A new missense mutation in the leptin gene causes mild obesity and hypogonadiem without affecting T cell responsiveness. J Clin Endocrinol Metab. 2010 Jun;95(6):2836-40. doi: 10.1210/jc.2009-2466.
- DFG - German Research Foundation
- Baden-Württemberg Foundation
- Boehringer Ingelheim Ulm University BioCenter (BIU)
- International Graduate School in Molecular Medicine Ulm (State of Baden-Württemberg)
For a list of publications please see PubMed.

